Why is galvanized reinforcing steel so effective?
1. Formation of the passive film
Like uncoated reinforcing steel, galvanized reinforcing steel also forms a protective passivating layer in concrete. Zinc in strongly alkaline solutions (pH 12.5 – 13.2) is passivated by the formation of a layer of adherent crystals of calcium hydroxyzincate – Ca[Zn(OH)3]2·2H2O). This reaction commences immediately on contact with the wet cement solution, forming a surface film which stabilizes the zinc and isolates it from the surrounding environment. In forming the passive layer, roughly 10 µm of the original pure zinc (eta) outer layer of the coating is consumed. The reaction with zinc ceases once the concrete hardens, and after 28 days when the concrete has developed its normal bond and compressive strength, the formation of the calcium hydroxyzincate layer results in galvanized reinforcing steel typically developing a higher bonded capacity and reduced slip strength characteristic when compared to uncoated reinforcing steel.
The properties of this passivating layer are key to galvanized reinforcing steel’s effectiveness in concrete, particularly its chemical stability at neutral pH and at high chloride concentrations.
2. Carbonation resistance
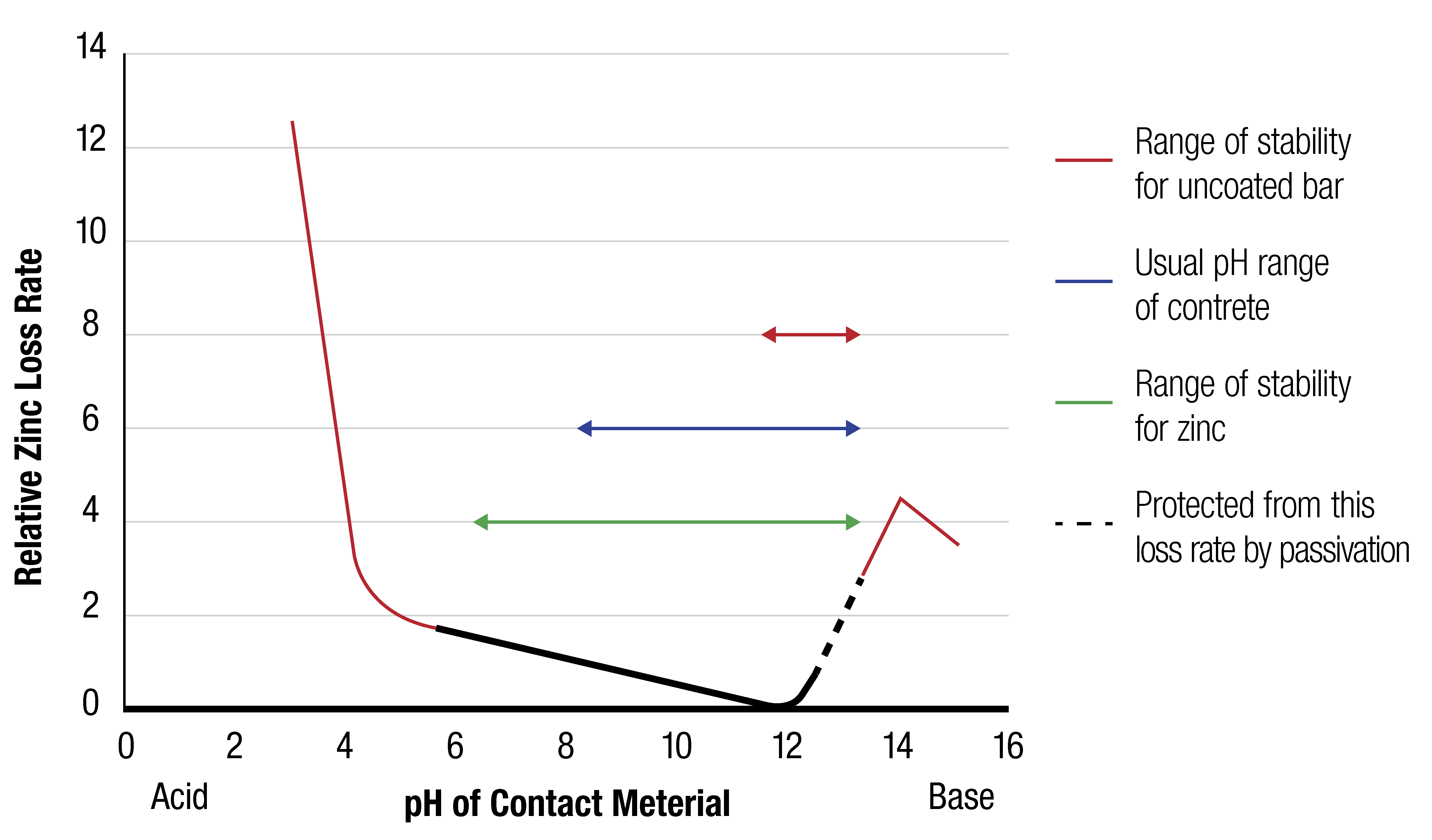
A hot dip galvanized coating has a very low corrosion rate over a wide range of pH values (pH 6 – 12.5). Because of this, galvanized reinforcing steel remains stable as the pH level of the concrete drops due to carbonation over its lifetime. Conversely uncoated reinforcing steel is only stable in a small range (pH 11.5 – 13.2) and will begin to corrode once the pH level of the concrete drops below 11.5.
In concrete with a pH between 12.5 and 13.2 the galvanized reinforcing steel is protected by the formation of the passive layer of calcium hydroxyzincate and this prevents the zinc from experiencing high loss rates in the highly alkali environment.
Over time the carbonation front migrates through the concrete mass, eventually reducing the pH to near neutral levels (pH 7). As the Figure below illustrates, galvanized reinforcing steel is therefore completely unaffected by the carbonation of concrete.

3. Resistance to chloride attack
The industry proposed chloride threshold for uncoated reinforcing steel is 0.06% by weight of concrete, based on a 20% chance of corrosion initiation. Galvanized reinforcing steel can tolerate chloride concentrations well above that which causes corrosion of uncoated reinforcing steel, due to the stability of the calcium hydroxyzincate film, and while there is no universal agreement, a literature review on the subject shows the chloride threshold of galvanized reinforcing steel to be 2 – 6 times higher than uncoated reinforcing steel. In general, a conservative value for the critical chloride threshold for galvanized reinforcing steel is considered 2 to 2.5 times than for uncoated reinforcing steel.
The rate of chloride diffusion through concrete slows down over time, so in practical terms the higher critical chloride threshold of galvanized reinforcing steel means that the time to corrosion initiation is much greater than for uncoated reinforcing steel – a least twice and in some reports, up to 10 times longer.
Because chloride attack is the single biggest cause of damage to reinforced concrete structures in worldwide infrastructure, it must be carefully considered in any durability plan. Hot dip galvanizing is a simple and cost-effective method to improve the chloride resistance, and therefore the durability, of concrete structures, and its performance relative to uncoated reinforcing steel can easily be modelled using conventional chloride diffusion models.
Chloride Diffusion Modelling
In durability planning, deterministic chloride diffusion models based on Ficks Second Law are often used to predict the durability of steel reinforced concrete structures. Variants of these models include Fick’s Second Law modified with an age factor (Bamforth, 2004), Luping and Gulikers Diffusion Model (2007) and the Time Weighted Average Diffusion Coefficient Model (RMS, 2018). All these models predict the time to corrosion initiation based on variables such as the surface chloride level (environment), concrete age, chloride diffusion coefficients, type and content of supplementary cementitious materials. A technical note on chloride durability modelling is available on the GAA website and shows that regardless of the deterministic model used, exposure environment, concrete mix or cover, the time to corrosion initiation for galvanized reinforcing steel is 2–10 times greater than that for uncoated reinforcing steel.
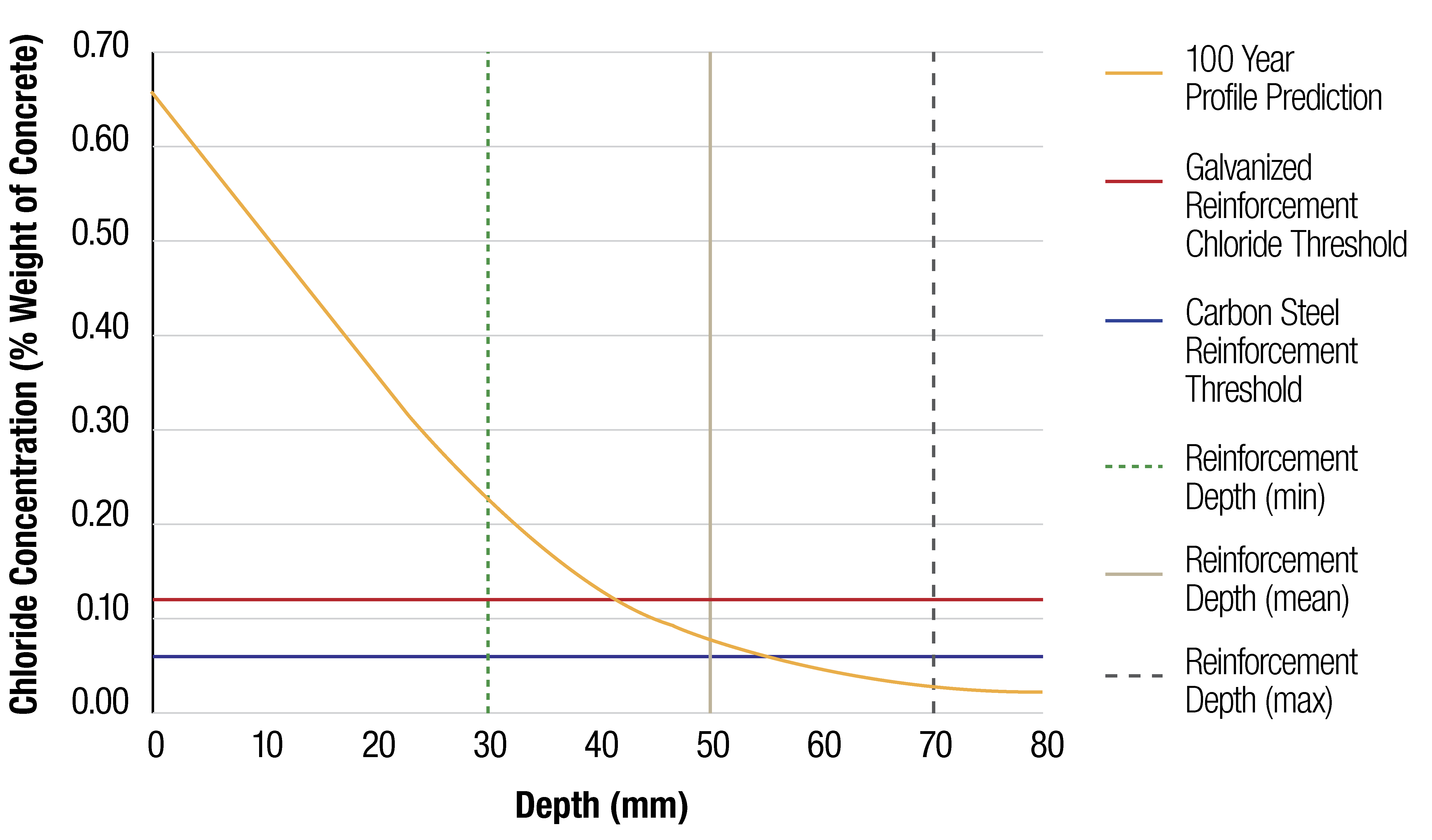
An example of this modelling below, which shows the chloride profile in a Marine Splash Zone at 100 years for different cement types, using the Luping and Gulikers Diffusion Model. Using a conservative value for the critical chloride threshold for galvanized reinforcing steel (X2), it can be shown that show that a cover reduction of at least 10 mm can be achieved. Alternately, maintaining the same cover, gives the asset owner a much greater certainty that the asset will meet or exceed its durability requirements.
Additionally, the time to corrosion initiation is likely to be much longer than the modelling indicates, as field studies indicate the chloride threshold in galvanized reinforcing steel is likely to be higher than the conservative value of 0.12% (by weight concrete) used herewith.
An important fact to note is that deterministic chloride diffusion modelling only models the time to corrosion initiation, which is independent of the thickness of the galvanized coating. And when considering the propagation phase of corrosion, during which the galvanized coating corrodes at a slower rate than steel, durability is further increased.
Hot dip galvanizing is a simple and cost-effective method to improve the chloride resistance, and therefore the durability, of concrete structures
4. Barrier protection
Another advantage provided by the zinc coating on galvanized reinforcing steel is that the hot dip galvanizing process provides complete coverage of all surfaces. This metallurgically bonded barrier protection acts as another defence between the steel and the atmosphere. This coupled with the coating’s excellent abrasion resistance and toughness makes it ideal for protecting reinforcing steel during transport to site and the construction stage of a project.
This metallurgically bonded barrier protection acts as another defence between steel and atmosphere.
5. Minimal disruption to concrete mass
Should corrosion initiation of the galvanized coating occur, the corrosion process enters the propagation phase. The resulting zinc corrosion products are fine and powder-like, expanding in volume only up to 1.3 times the original zinc volume (compared to uncoated reinforcing steel which expands up to 7 times the original steel volume).
The zinc corrosion products are also more soluble in the alkali pore water and diffuse away from the reinforcing steel and into the concrete matrix, unlike iron corrosion products which won’t migrate away from the reinforcing steel until after concrete cracking has occurred (Zhao & Jin). This avoids the build-up of internal pressures which lead to concrete cracking and spalling.
Furthermore, the addition of the microscopic corrosion products to the concrete matrix decreases its permeability by filling pores and voids, thus slowing the supply of aggressive species from the concrete surface to the reinforcing steel. The result of this process is a significant increase in time for the propagation phase of corrosion and a corresponding delay in the time to initiation of cracking of the concrete.
6. Sacrificial protection
The galvanic series of metals is a list of metals and alloys arranged according to their relative potentials in each environment. The picture (below) shows a series of metals arranged in order of electrochemical activity in seawater (the electrolyte). Metals to the left of the scale provide cathodic or sacrificial protection to the metals on the right.
Zinc is anodic to steel; therefore, the galvanized coating will provide cathodic protection to exposed steel. When zinc and steel are connected in the presence of an electrolyte, the zinc is slowly consumed while the steel is protected. Zinc’s sacrificial action offers protection to the steel in the event of local coating discontinuities and as the result of severe surface abrasion during rough handling or job site erection.


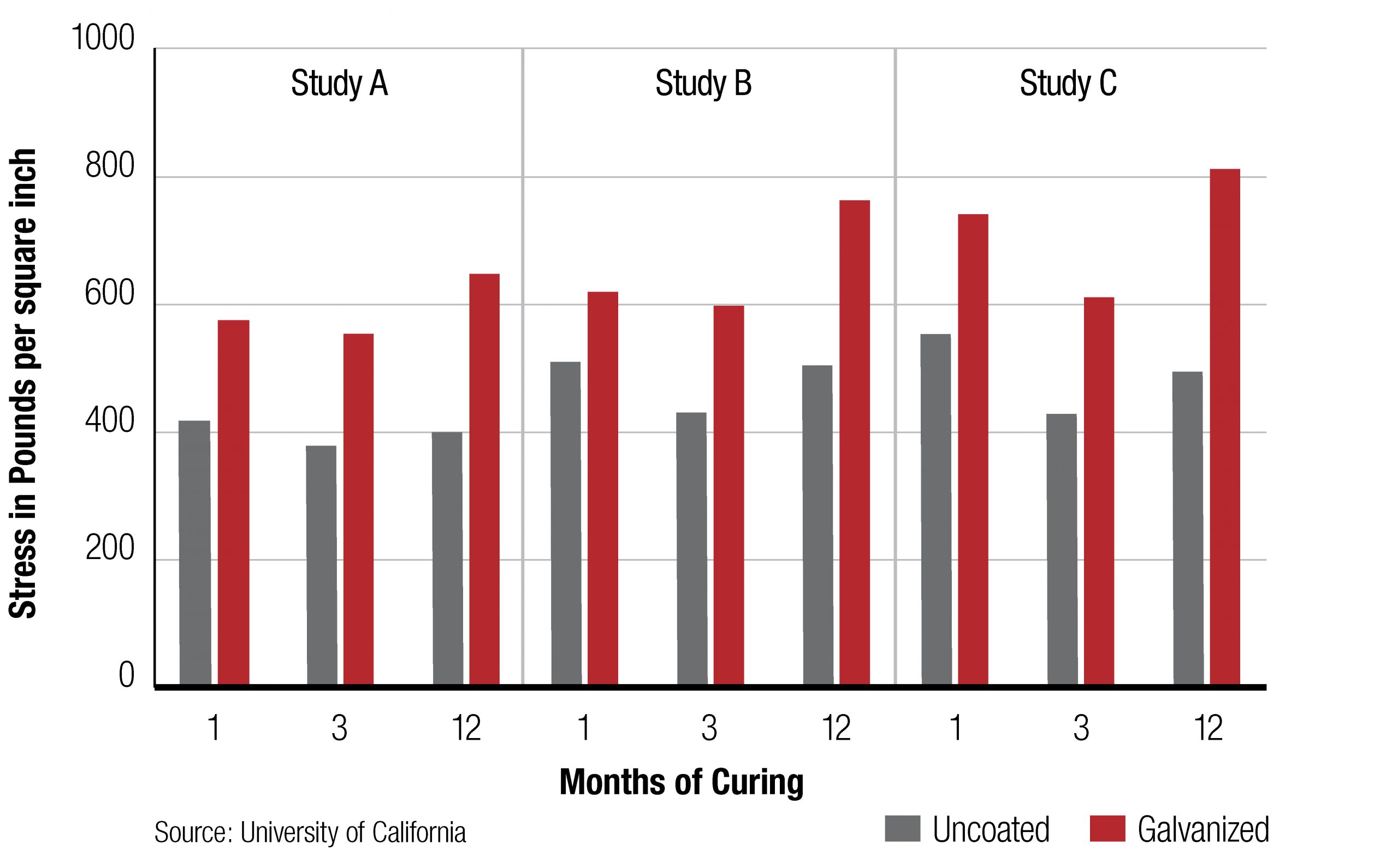
7. Bond strength
There is extensive evidence that supports the galvanized reinforcing steel’s superior bond strength characteristics compared to uncoated reinforcing steel. The bond strength formed is closely linked to the formation of the passive calcium hydroxyzincate film and, while it has been reported in accelerated tests that the bond strength of galvanized reinforcing steel lags behind that of uncoated reinforcing steel, this effect only lasts for the first 1 – 2 weeks and is related to the initial reaction of zinc to the highly alkaline conditions. After 28 days, when the concrete has developed its normal bond and compressive strength, the galvanized reinforcing steel will develop a higher bond capacity and reduced slip strength characteristic when compared to uncoated reinforcing steel. This is because of the precipitation of the calcium hydroxyzincate film at the reinforcing steel concrete interface.