Corrosion of uncoated reinforcing steel
The highly alkaline environment of concrete allows conventional uncoated reinforcing steel to develop a stable, passive iron oxide film on its surface, which protects the steel from corrosion. However, concrete is an inhomogeneous material, mainly composed of the hydration products of cement (cement paste), sand and aggregates. The inherent porosity of cured concrete provides a pathway for the diffusion of gaseous and aqueous species which, over time, can break down the passivity of the steel and initiate corrosion.
Corrosion of conventional reinforcing steel in concrete is initiated when the protective oxide layer on its surface is depassivated. Depassivation can occur by either 1 of 2 separate mechanisms:
1. Carbonation of the concrete, or
2. Chloride induced corrosion.
Once corrosion of the reinforcing steel is initiated, corrosion products begin to form on the surface of the reinforcing steel. These products are substantially more voluminous than the original steel (up to 7x increase in volume for carbon steel). This increase in volume from the steel corrosion applies significant tensile stresses to the concrete and eventually causes the formation and propagation of cracks. These cracks in turn provide a pathway for the rapid ingress of aggressive agents to the reinforcing steel, which will accelerate the steel corrosion process, thereby causing damage such as the delamination or spalling of the concrete cover.
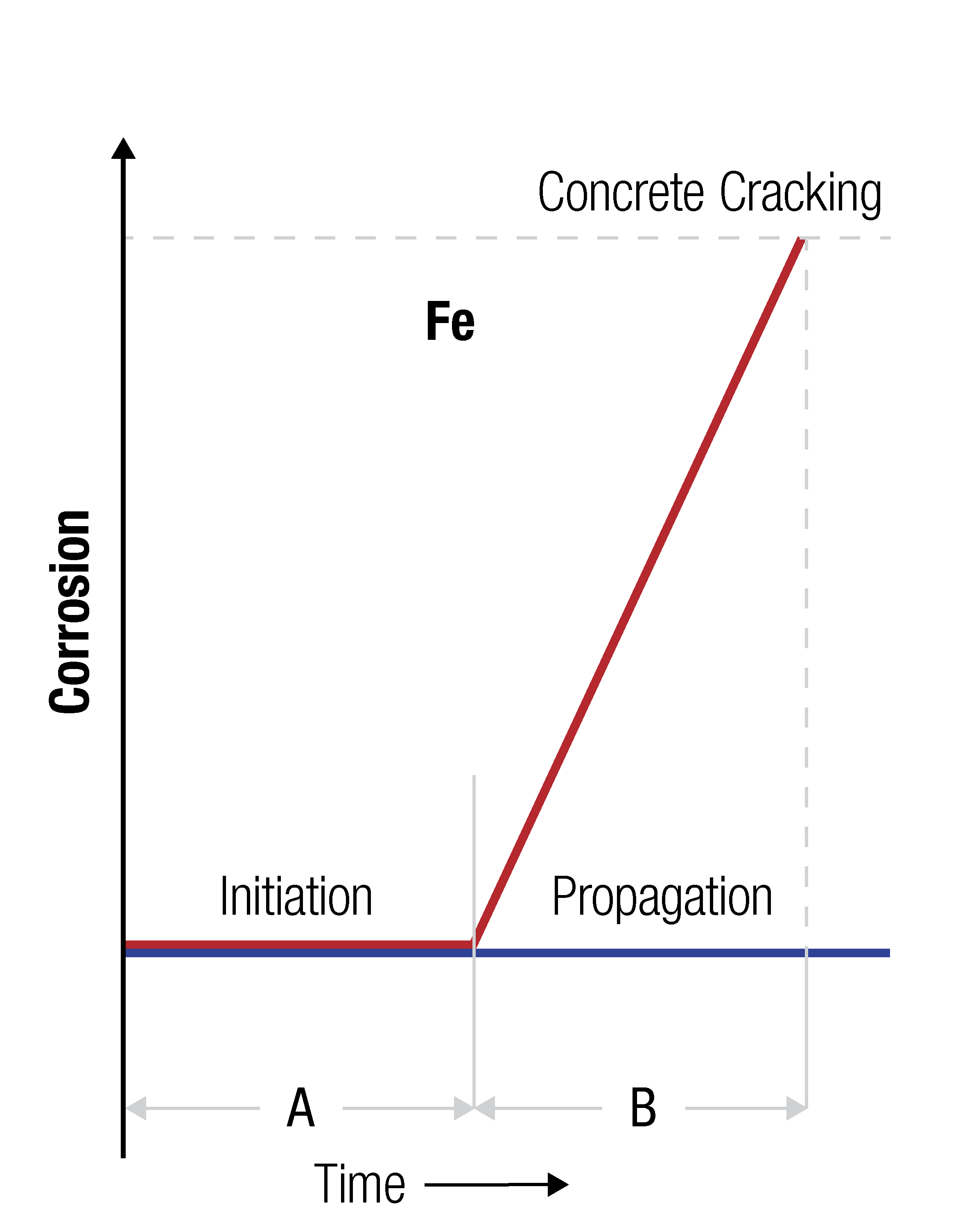
The corrosion process for uncoated reinforcing steel is shown graphically in the adapted Tuutti Model below, where:
A. The initiation stage – the period in which the reinforcing steel remains passivated (until the point A-B).
B. The propagation stage – Destruction of the passive layer on the reinforcing steel has occurred, and the steel is actively corroding. At the end of this time cracking and spalling of the concrete occurs.
The corrosion process is most commonly initiated by either neutralization of the environment surrounding the reinforcing steel, e.g. carbonation, or activation of the surface by strongly corrosive anions, e.g. chlorides. The time to corrosion initiation is determined by the concentration and flow rate of penetrating substances into the concrete cover and by the threshold concentration required for corrosion to start.
Carbonation
Carbonation is a natural process that occurs when the high alkalinity of the cover concrete is neutralized due to a reaction with atmospheric carbon dioxide. Over time the carbonation front migrates through the concrete mass eventually reducing the pH to near neutral levels (pH 7). As the pH of the concrete drops, the reinforcing steel inside the concrete become more susceptible to corrosion.
Typical characteristics of the carbonation of concrete are:
- The rate of the carbonation reaction
is lower further into the concrete (the pH profile) - The depth of carbonation depends on the concrete permeability and cracks, voids, and pores
- Once the pH of the concrete drops to less than 11.5 the reinforcing steel will begin to corrode
- The effect and rate of the neutralization is stronger when sulphur dioxide (SO2) and nitrogen dioxide (NO2) react with water to form highly acidic solutions. These chemicals are of higher concentration in the environment in industrial areas where they are introduced to the atmosphere via emissions.
Based on field measurements, the quality of the concrete is critical in reducing the effects of carbonation. Testing has revealed that:
- In quality structural concrete (40–50 MPa), carbonation may be seen up to as little as 5–10 mm after 20 years of atmospheric exposure (for example, structural elements of buildings in an urban environment)
- In poor quality concrete (≈20 MPa), full carbonation of 200 mm thick wall panels (from both sides) occurs in 5 – 8 years (for example, low cost public housing buildings).
Chloride attack
Chloride induced corrosion is the single biggest cause of reinforcing steel corrosion and consequential damage to reinforced concrete structures world-wide. Chloride ions can migrate through concrete and build up to the levels required to cause depassivation of the protective film on the surface of the reinforcing steel, thereby initiating corrosion. The chloride ions activate the surface of the steel to form an anode with the remaining passivated surface being the cathode. Chloride ions then attack the ferrous oxide, forming complexes that move away from the steel and become rust. Newly exposed iron atoms form more ferrous oxides, thus continuing the corrosion process.
Chlorides enter the concrete via:
- Contaminated aggregates, marine sands, and admixtures
- Brackish or saltwater used for mixing and/or curing
- Exposure to marine and coastal environments
- Use of de-icing salts.
Chlorides migrate through the concrete over time by a process called diffusion, thereby increasing the chloride concentration at the reinforcing steel surface.
A chloride threshold of 0.2 – 0.4% of the cement content (or 0.6 kg/m3 of concrete) has been identified as the range that uncoated reinforcing steel can begin to corrode.






