Durability under water
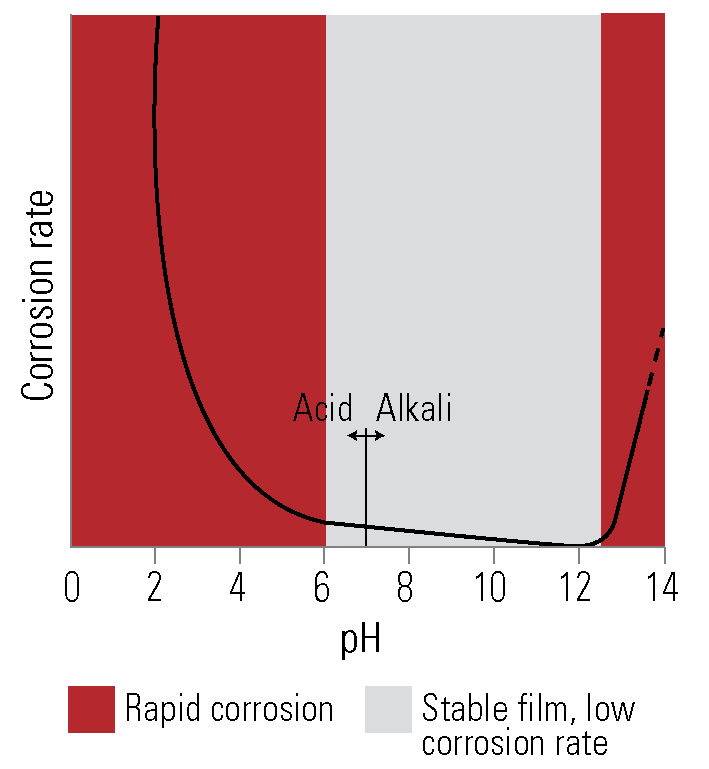
General. The corrosion rate of zinc under immersed conditions can be high in acidic solutions below pH 6 and alkaline solutions above pH 12.5. Between these limits the rate of corrosion is much lower. Time of exposure, temperature, motion, and fluid agitation all influence the corrosion of zinc in water.
In mains supply water of pH 6 to pH 8, calcium and bicarbonate ions are normally present, which leads to precipitation of an adherent calcium carbonate scale on the galvanized coating, together with zinc corrosion products, forming an impervious layer. When sufficiently dense, this layer virtually stops corrosion of the coating, resulting in very long life in many water systems.
Other factors may interfere with this scale deposition. If the water has a high concentration of uncombined carbon dioxide, the protective scale is not formed, and full protection never develops. The characteristics of the water supply should be considered in the design of water systems. The presence of even small quantities of dissolved copper in the order of 0.1 parts per million in the water may cause corrosion by rapid pitting.
In unfavourable waters, galvanized steel may require the added protection of galvanic anodes or suitable paint coatings.
It is also important to recognise that the “Plumbing Code” does not permit the use of galvanized pipes for drinking water but does allow them for firefighting systems. Some states of Australia have differing rules in relation to the galvanized steels to collect rainwater (e.g., storage tanks) and the subsequent use.
Pure water. When newly galvanized articles are immersed in pure water, such as rainwater, there are no dissolved salts present to form the film of insoluble compounds which normally protects the coating from further action. Where practical this condition can be corrected by the addition of controlled amounts of salts to the water during initial immersion. Most natural waters contain sufficient dissolved salts to prevent initial attack and galvanized tanks and equipment give excellent service.
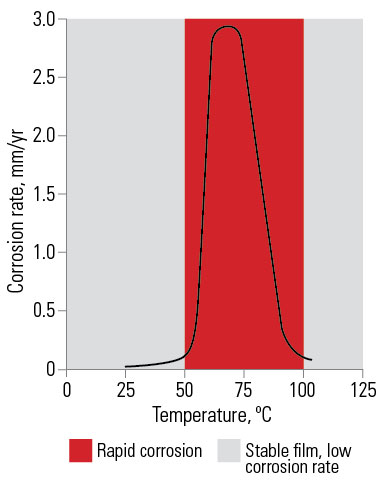
Effect of water temperature. In cold water of normal composition galvanized coatings are most effective and the rate of consumption of the galvanized coating is very low. This has resulted in almost universal use of galvanized steel for tanks for water storage and transport.
At about 60°C to 65°C the rate of corrosion of galvanized coatings increases and continued corrosion resistance depends on early formation of adequate non-flaking scale. Hard water in hot water systems will deposit a scale of calcium and magnesium carbonates on the galvanized surface, nullifying the temperature effect. Soft water may not deposit a protective scale. In such cases, galvanized coatings are unsuitable for hot water systems.
Sea water. Galvanized coatings perform relatively well in submerged sea water conditions which are severely corrosive to most protective systems. Dissolved salts present in sea water react with zinc to form a protective layer minimizing corrosive action.
The addition of a suitable paint system or wrap is recommended in areas of severe sea water exposure, particularly in tidal areas and splash zones.