Bimetallic corrosion
Bimetallic corrosion (also known as galvanic or dissimilar metal corrosion) with resulting rapid consumption of the zinc coating is likely if a galvanized article is installed in contact with brass or copper, particularly in a moist environment. Contact between aluminium and other galvanized surfaces is normally satisfactory.
Bimetallic corrosion occurs for the same electrochemical reasons as those by which zinc provides cathodic protection for steel but the rate of consumption of zinc coatings by galvanic corrosion may be extremely high.
Galvanized surfaces in contact with other metals
Bimetallic corrosion requires electrical contact in the presence of an electrolyte and cannot occur in the absence of these factors. Generally hot dip galvanized steel performs well in contact with most common engineering metals when in an atmospheric environment (Table 7), provided the ratio of hot dip galvanized steel to other metals is high. Conversely in immersed conditions, the effect of bimetallic corrosion is significantly increased, and some form of isolation will normally be required. For maximum corrosion resistance under conditions of extreme humidity, overlapping galvanized surfaces should be isolated from each other by the application of an inhibitive jointing compound or suitable paint. Galvanized surfaces in contact with other materials may also require isolation.
Table 7 Performance of galvanized steel in contact with other metals
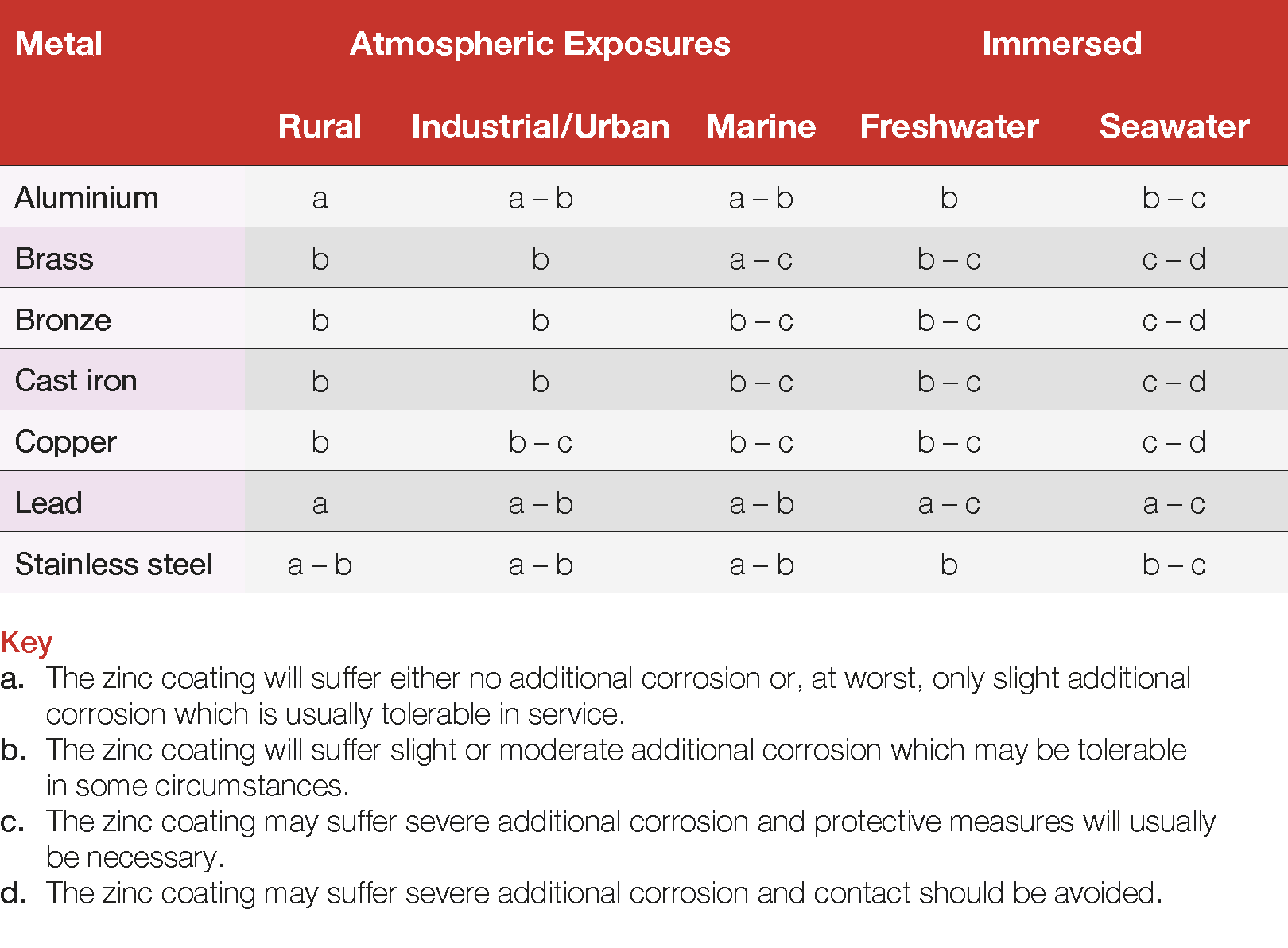
Practical experience suggests that, where the surface area ratio of zinc to other metal is high and a rating of “a” or “a – b” is indicated, little or no additional corrosion will take place because of the contact. However, where the ratio of surface areas is reduced or the rating is higher, some form of insulation may be required.
The following guidance relates to specific applications concerning zinc coated steelwork in contact with the designated metal or alloy.
Aluminium – The severity of increase in bimetallic corrosion due to atmospheric contact with aluminium is relatively low. Galvanized members in contact with aluminium conductors may require the use of an electrically conductive compound at joint faces to repel moisture and inhibit corrosion. One application where galvanized steel and aluminium are used in conjunction with one another is aluminium cladding. In this instance, isolation is advised due to the large surface area of the aluminium panels.
Copper – Due to the large potential set up by contact between zinc coated steel and copper and copper-containing alloys, electrical isolation is always advised, even in an atmospheric environment. Run-off water from copper surfaces frequently contains small quantities of dissolved copper sufficient to cause attack and rapid deterioration of a zinc coating through chemical deposition of copper. Where possible, design should avoid run-off of water from copper onto zinc coated articles. Joint faces should be insulated using non-conducting gaskets or mastics and connections should be made with insulating grommet-type fasteners. The design should be arranged so that water flows from the galvanized surface onto the brass or copper surface and not the reverse.
Lead – Potential for bimetallic corrosion with lead is low in an atmospheric environment and no problems have been reported concerning, for example, the use of lead flashing with zinc or zinc coated products and the use of lead in fixing galvanized posts.
Stainless steel – The most common use of stainless steel with zinc coated steel is in the form of nuts and bolts in an atmospheric environment. Given the low potential for bimetallic corrosion and the small surface area of stainless-steel fasteners, bimetallic corrosion would not normally be an issue although, as always, best practice remains isolation using insulating washers.




